Materials scientists from UC Santa Barbara uncover source of degradation in sodium batteries
View the complete news release at: https://www.news.ucsb.edu/2019/019555/toward-better-battery
(Santa Barbara, Calif.) — Batteries power our lives: we rely on them to keep our cell phones and laptops buzzing and our hybrid and electric cars on the road. But ever-increasing adoption of the most commonly used lithium-ion batteries may actually lead to increased cost and potential shortages of lithium — which is why sodium-ion batteries are being researched intensely as a possible replacement. They perform well, and sodium, an alkali metal closely related to lithium, is cheap and abundant.
The challenge? Sodium-ion batteries have shorter lifetimes than their lithium-based siblings.
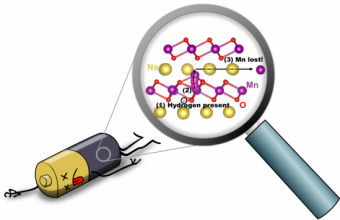
Now, UC Santa Barbara computational materials scientist Chris Van de Walle and colleagues have uncovered a reason for this loss of capacity in sodium batteries: the unintended presence of hydrogen, which leads to degradation of the battery electrode. Van de Walle and co-authors Zhen Zhu and Hartwin Peelaers published their findings in the journal Chemistry of Material



